UNIT PROCESS industrial chemistry topic easy notes




UNIT PROCESSES
Unit process involves principle chemical conversions leading to synthesis of various useful product and provide basic information regarding the reaction temperature and pressure, extent of chemical conversions and yield of product of reaction nature of reaction whether endothermic or exothermic, type of catalyst used.
Some unit processes are followings:
➤ NITRATION
Nitration involves the introduction of one or more nitro groups into reacting molecules using various nitrating agents like fuming, concentrated, aqueous nitric acid mixture of nitric acid and sulphuric acid in batch or continuous process. Nitration products find wide application in chemical industry as solvent, dyestuff, pharmaceuticals, explosive, chemical intermediates.
➤ HALOGENATION
Halogens involve introduction of one or more halogen groups into a organic compound for making various chlorine, bromine, iodine, fluorine organic derivatives. All though chlorine derivatives find larger application. however some of the bromine and fluorine derivatives are also important. Various chlorinating agents are chlorine, HCl, phosgene sulfuric chloride, hypochlorite, bromination, bromine, hydrobromic acid, bromide, bromated, alkaline hypobromites. In iodination iodine, hydroiodic acid and alkali hypoiodites.
Typical important chemicals are chlorinated products: Ethylene dichloride, chlorinated methanes Chloroform, Carbon tetra chloride etc) Chlorinate ethane, Chloro propane, chloro butanes, chloroparaffins, chlorination of acetaldehyde (Chloral), alkyl halhides, Chlorobenzene, Ethylene diiodide, Chloroflurocarbon (CFCs).
➤ SULPHONATION & SULPHATION
Sulphonation involves the introduction of sulphonic acid group or corresponding salt like sulphonyl halide into a organic compound while sulphationinvolves introduction of-OSO2OH or -SO4-. Various sulphonating agents are sulphur trioxide and compounds, sulphurdixide, sulphoalkylating agents. Some of the sulphaming agents are sulphamic acid. Apart from sulfonation and sulphamate sulpho chlorinated, sulfoxidation is also used. Typical application of sulphonation and sulphation are production of lingo sulphonates, linear alkyl benzene sulphonate. Toluene sulphonates, phenolic sulphonates, chlorosulphonicacd, sulphamates for production of herbicide. sweetening agent (sidiumcyclohexysulphamate). Oil soluble sulphonate, saccharin.
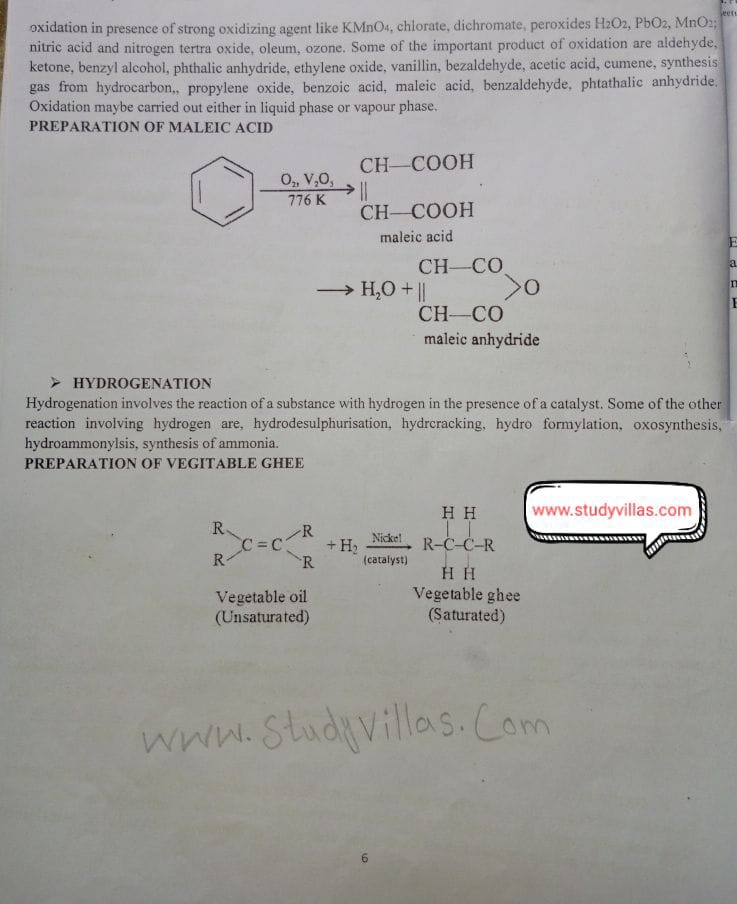
➤ OXIDATION
Oxidation used extensively in the organic chemical industry for the manufacture of a large number of chemicals Oxidation using oxygen, are combinations of various reactions like oxidation via dehydrogenation using oxygen dehydrogenation and the introduction of oxygen and destruction of carbon, partial oxidation, peroxidation
oxidation in presence of strong oxidizing agent like KMnO4, chlorate, dichromate, peroxides H2O2, PbO2. MnO:: nitric acid and nitrogen tertra oxide, oleum, ozone. Some of the important product of oxidation are aldehyde, ketone, benzyl alcohol, phthalic anhydride, ethylene oxide, vanillin, bezaldehyde, acetic acid, cumene, synthesis gas from hydrocarbon., propylene oxide, benzoic acid, maleic acid, benzaldehyde, phtathalic anhydride. Oxidation maybe carried out either in liquid phase or vapour phase.
➤ HYDROGENATION
Hydrogenation involves the reaction of a substance with hydrogen in the presence of a catalyst. Some of the other reaction involving hydrogen are, hydrodesulphurisation, hydrcracking, hydro formylation, oxosynthesis, hydroammonylsis, synthesis of ammonia.
➤ ESTERIFICATION
Esterification is an important unit process in the manufacture of polyethylene terephathalate, methyl metha acrylate, cellulose ester in viscose rayon manufacture (xanthation of alkali cellulose with carbon disulphide), nitroglycerine.
➤ HYDROLYSIS
Hydrolysis is used both in inorganic and organic chemical industry. Typical application is in oil and fats industry during soap manufacture where hydrolysis of fats are carried out to obtain fatty acid and glycerol followed by addition of sodium hydroxide to form soap. Other application is in the manufacture of amyl alcohols. Some of the major product using hydrogen is ethylene from acetylene, methanol, propanol, butanol, production of alcohol from olefins (eg. Ethanol from ethylene).
Various types of hydrolysis reaction may be pure hydrolysis, hydrolysis with aqueous acid or alkali, dilute or concentrated, alkali fusion, hydrolysis with enzyme and catalyst.
